The Nov. 15, 2013 newsletter of America's leading informational website on aging issues, TodaysSeniorsNetwork, is now online. Stay informed on the many issues facing an aging America. http://app.flashissue.com/newsletters/americas-leading-informational-website-on-aging-issues-7

Making the case for personal importation of safe, affordable prescription medicines from licensed, registered pharmacies in Tier One Countries. Rx for American Health is published by Daniel Hines, an international award-winning communicator with five decades of experience, and the publisher of www.TodaysSeniorsNetwork.com and www.BoomersNewsOnline.com. He also works with progressive senior advocacy groups across the nation to promote the health and well-being of America’s aging population.
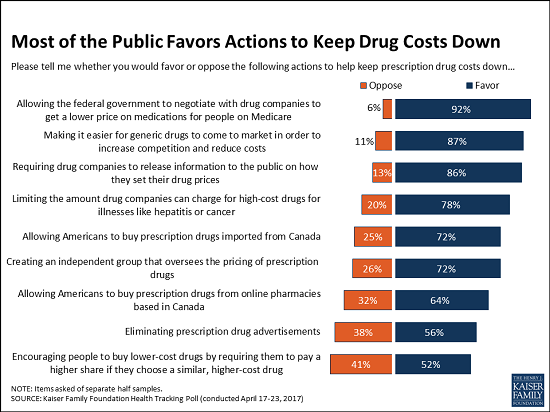
Kaiser Poll Show Support for Personal Imporatation
Saturday, November 16, 2013
Thursday, October 31, 2013
America's Leading Informational Source about Aging
America's Leading Informational Source about Aging
America's Seniors at TodaysSeniorsNetwork's latest newsletter is now online. Click http://flsh.is/1aWNfsf to stay informed about these important issues of Aging in America. Your one-stop source for all issues affecting elderly, caregivers, policy-makers, advocates.
America's Seniors at TodaysSeniorsNetwork's latest newsletter is now online. Click http://flsh.is/1aWNfsf to stay informed about these important issues of Aging in America. Your one-stop source for all issues affecting elderly, caregivers, policy-makers, advocates.
Saturday, October 26, 2013
Friday, March 15, 2013
Pharma practices ‘disingenuous connectivity’ in attacks upon personal importation
Pharma, and its trade group PhRMA,
are employing a strategy that is best described as ‘disingenuous connectivity’
in its latest strategies against personal importation of prescription medicines.
The strategy-- attempting to remove itself as the focus of attacks upon personal importation of safe, affordable medicines, from licensed, registered pharmacies in Tier One Countries whose standards of safety and efficacy for prescription medicines meet or exceed those of the US.
Why does this constitute being disingeous? Let's look at the Dictionary definition:
This is what Pharma is doing in establishing relationships
and connections with erstwhile and commendable interest groups to
persuade them to adopt the admirable goal of prescription drug safety as a part of their
mission, but one in which any imported medicines are by definition of being
imported are, in and of themselves, ‘unsafe’.
Some examples: The Institute of Medicine,
a national Non-Governmental
Organization designed to
provide national advice on health and medical issues and to develop programs for
patient safety, have, in response to a
request from the Food and Drug Administration
for a study on drug safety issued a
statement calling for increased tracking and tracing of the worldwide
pharmaceutical chain of custody.
The IOM statement does base its
appeal on recognition of the problems of admittedly bogus pharmacies and counterfeit
medicines. It is disturbing in other
ways, however, not the least of which is
that IOM has dropped the use of the word
‘counterfeit’ to describe fake medicines, choosing instead to restrict the term
to encroachment of intellectual
property rights, a Pharma goal in its failed PIPA and SOPA pushes.
Another example: In a recent article in the Journal of Nursing,
the nurse community is urged to assist in counseling patients on drug safety,
using standards that limit the application of a ‘safe pharmacy’ or source of
personally imported medicines to only those in the US.
The FDA is a major player in this
effort. Its new web site ‘identifying’
what it describes as ‘unsafe’ pharmacies uses the VIPPS program as a
resource, an embrace that reflects the success of the National
Boards of Pharmacy Verified Internet Pharmacy Practice Sites (VIPPS) program
and opponents of personal importation to provide what might be a ‘gateway’
service designed to allow FDA and Pharma to claim, because there continues to
be legislation empowering the FDA to contract selected services to privately
sourced, third-party groups in the private sector to conduct safety oversight
that normally would be a part of the FDA responsibility within the limits
determined by Congress.
As to prove the case, the Journal of
Nursing refers nurses and patients to the FDA site and the VIPPS program as the
‘standards’ for safety.
To imply that all medicines from licensed,
registered pharmacies outside the US are unsafe does not stand in the light of
facts. Many countries, especially those in Tier One Countries do have excellent
standards of safety. Add to this that
virtually all prescription medicines sold in the US are manufactured at plants
outside this country, meaning that even those medicines sold in NABP
pharmacies, are likely imported into the US.
The target of these programs is not prescription
medicines that might be ordered from clearly identifiable bogus pharmacies on
the Internet, but those safe sources from outside the US that provide as many
as two million Americans access to safe, affordable prescription medicines that
would otherwise be denied to them because of the predatory pricing practices of
Pharma that have made the US a safe haven for the highest prescription drug
prices in the industrialized world.
No one disputes the need to ensure
that Americans are made aware of the potential danger of bogus pharmacies, and
providing Americans with the tools to identify such pharmacies. There are ample
guidelines on identifying guidelines to ensure safety based on more than a
decade of safe use of personally imported prescription medicines.
For more than 12 years,
seniors’ advocacy groups and others have made the case that access to safe,
affordable medicines via personal importation is a matter of fact.
Supporters of personal importation have published
stories, guidelines, and have developed materials to help identify the validity
of a pharmacy. The US Congress has repeatedly passed legislation in support
of personal importation only to see it turned aside by ‘poison pill’ amendments
brought forth by Congressional supporters of Pharma.
Even the FDA has taken actions that
validate the claims of supporters of personal importation that the regulatory
agencies of Tier One Countries meet or exceed those of the US and should
therefore be the basis of approval for personally imported medicines, by moving
to allow a form of reciprocity in the oversight and manufacture of
ingredients for prescription medicines by regulatory agencies in countries
outside the US.
The answer: this just another example of the disingenuous
strategies of Pharma and others.
There are more:
PhRMA
has launched a series of efforts to attain goals that it was denied by the
legislative process, including what is cited as patent and trademark
protection but really was nothing more than attempting to co-opt legislation by
shaping it in a manner that reflected its goals of deterring personal importation.
The examples below amplify the case
that Pharma is indeed engaged in a full scale attack upon personal importation
with no concern for the vital lifeline it provides to untold numbers of
Americans, or the fiscal impact to citizens being denied access to affordable
medicines:
The goal is the silencing of
seniors’ advocates and others who support the benefits that access to such a
vital lifeline and savings from prescription medicines offered by personal
importation.
The strategy-- attempting to remove itself as the focus of attacks upon personal importation of safe, affordable medicines, from licensed, registered pharmacies in Tier One Countries whose standards of safety and efficacy for prescription medicines meet or exceed those of the US.
Why does this constitute being disingeous? Let's look at the Dictionary definition:
‘Witholding known information or giving a false
impression of sincerity or simplicity’’.
· The drafting
of a bill by the Senate Health Committee and House Energy and Commerce
Committee in October 2012, written by staff members, free of any input from
stakeholders. The ‘draft’ established the framework for the empowering of
the FDA to enter into third-party relationships with the private sector
granting the authority mentioned in the paragraph above. The 118-page
draft was not even submitted for consideration. The question must be
asked: Why not? Is it still in someone’s desk drawer waiting to be pulled
out for introduction in the 113th Congress,
free of any opportunity for input from stakeholders? The language also focuses
on ‘potentially’ unsafe medicines, a particularly troublesome term since there
are no standards of what constitutes ‘potentially’ except for several mentions
of ‘misbranded’ (read personally imported) medicines.
· The FDA
Reauthorization bill (PDUFA) calls
for Rules Promulgation in the next two years. Section 708 empowers the
seizure of medicines dispensed by pharmacies in countries outside the US for
Americans’ use by granting the power of seizure to Customs, Homeland Security,
and Border Agents. Questions that must be asked and answered include but are
not limited to:
o When
will FDA communicate the schedule on Rules Promulgation;
o How will it
ensure that all persons and groups desiring to make comments are notified?
o The
challenge: the language as written gives authority for the Secretaries of
HHS and Homeland Security to make seizures by Customs and destroy
them. In a disturbing aside, Senator Bill Nelson (D-FL) , chair of the
Senate Select Committee on Aging, and a supporter of personal importation,
tells a senior advocate in Florida that he believes the action in 2007 when he
and Senator David Vitter (R-LA) led legislation that ended FDA-Customs
collusion resulting in seizures of personally imported medicines , is a
precedent that will preclude such action now. Unfortunately, he is
incorrect.
· FDA
launches a website in an effort to ‘identify’ what it describes as ‘safe
pharmacies’, all within the US, again using the VIPPS model;
· The Center for Safe Internet Pharmacies ‘launches’, a year after its
founding. The timing of the launch seems to be a part of a communications
effort to attain a critical mass to determine the ‘message’ that the definition
of safe pharmacies of safe pharmacies is applicable only to those in the
US. Again, the VIPPS model comes into
play;
· FDA issues a
series of news releases, including statements from FDA Commission Margaret
Hamburg, about the need for new initiatives in the chain of custody to
guarantee the safety of the prescription medicine supply. This comes only
months after more than 60 people dying from unsafe medicines from a
Massachusetts compounding pharmacy.
· The problem
with the counterfeit Avastin (bogus?) continues to be a mainstay of FDA
releases. The identification of potentially dangerous medicines is a
commendable goal and important to protecting Americans, but there is no record
of anyone becoming ill of dying from taking the Avastin. The Federal
government has commendably been diligent in its prosecution of offenders, many
of whom are physicians.
· The
President of Eli Lily, angered by the refusal of Canadian authorities to
approve a patent, suggests in a prepared statement that Lily is granted a
degree of sovereignty equal to that of Canada due to the North American Free
Trade Agreement, and , that companies such as Lily (and other Pharma
companies?) have such a standing in not only NAFTA, but future trade
agreements. What does this say about the sovereignty of a country like Canada...or even the
US...and its authority to conduct its governmental functions?
· PhRMA apparently has returned to the issue of controlling
the Internet. But, apparently burned by its SOPA debacle, this time,
Pharma has the NABP acting on its behalf as it seeks a new domain designation for
.pharmacy. The
.pharmacy designation would be specific for US-based pharmacies only, would
disable Americans from having access to Internet pharmacies in other countries
(including Canada), and would utilize, yes, the VIPPS list to determine
approved pharmacies, in effect granting PhRMA the victory it was denied in the
PIPA and SOPA battles. Of interest, in its application for the .pharmacy
designation, NABP declares that FDA, Pharma, other groups will conduct a vigorous
communications and educational campaign on the significance of the new domain
designation. This is a move to encroach upon the freedom of the Internet
and to attempt to utilize .pharmacy for the special interests of NABP and
Pharma. It should receive the same vigorous opposition as the SOPA
boycott.
Look for the pattern to hold true.
The FDA is faced with budget cuts, plus Sequestration, Pharma is desperate to
circumvent the openness of the legislative process with its demands for
hearings, testimony, and, public statements from stake holders.
Pharma and its allies are
opting for passage of administrative rules that the rules makers will adopt
citing that they either has the power to make such decisions, and which it will
attempt to validate through the passage of vaguely written ‘drafts’ crafted
behind closed doors.
It provides us with a new
phrase to describe what we are witnessing: disingenuous
connectivity. It is time for Congress to pull the plug.
Wednesday, January 23, 2013
Helping Doctors Communicate Better When Prescribing Meds
Newswise — When it comes to
prescribing medications to their patients, physicians could use a dose of extra
training, according to a new study led by a UCLA researcher.
In
previous studies, Dr. Derjung Tarn and her colleagues found that when doctors
prescribed medicines, the information they provided to patients was spotty at
best, they rarely addressed the cost of medications and they didn't adequately
monitor their patients' medication adherence.
The logical
next step, Tarn said, was to devise an intervention aimed at improving how
physicians communicate to their patients five basic facts about a prescribed
medication: the medication's name, its purpose, the directions for its use, the
duration of use and the potential side effects.
And it appears to have worked.
Tarn and
her co-researchers found that physicians who completed the training
demonstrated a significant improvement in how they communicated this crucial
information.
Compared to a control group that didn't receive the training,
these doctors discussed at least one additional topic out of the five — and
they sometimes went beyond the basics, touching on other pertinent facts about
medications that are important for patients to know.
The
intervention is described in the January issue of the journal Annals of Family Medicine.
"We
were pleasantly surprised to see that a simple intervention was effective in
improving the content of discussions," said Tarn, the study's lead author
and assistant professor of family medicine at the David Geffen School of
Medicine at UCLA.
The
researchers conducted a controlled clinical trial between February 2009 and
February 2010 with 27 primary care physicians and 256 patients.
The training
consisted of a one-hour interactive educational session that encouraged doctors
to communicate the five basic facts about prescribed medications.
The
researchers also gave the participating patients a flier listing the five
facts. In addition, they recorded the audio of the physician–patient
interactions.
The success of the physicians' communication of the key facts to
patients was measured using the Medication Communication Index, or MCI.
The
researchers found that the mean MCI for the physicians in the intervention
group was 3.95 out of five, compared with 2.86 for those physicians who didn't
receive the training. The intervention-group doctors also received higher
ratings from their patients on how they communicated information about
medications than did the physicians in the control group.
And,
significantly, the training resulted in more than just better communication
about the medications the physicians prescribed, according to the study.
"Interestingly,
higher MCI scores also were associated with more reports of communication about
topics not directly included in the intervention," the researchers write.
"For example, the intervention encouraged physicians to discuss potential
medication side effects with patients, but patients also reported better
communication about the risk of experiencing side effects and what to do if
side effects occurred."
The study
has some limitations. Patients were predominantly white, most had at least some
college education, and there were more Hispanics than African Americans.
Also,
having an audio recorder in the examination room may have enhanced
communication for physicians in the intervention group more than for those in
the control group, who were unaware of what the researchers were studying. In
addition, the researchers didn't examine the doctors' style of communication,
and they don't know if any additional time spent talking about new
prescriptions might have detracted from conversations about other topics.
Still, the
study suggests "that a brief, practical intervention can improve physician
communication about newly prescribed medications in ways that affect
patients," the researchers write. "The intervention should be tested
for its clinical impact."
Tarn's
co-researchers on the study were Chi-hong Tseng and Neil S. Wenger of UCLA,
Debora A. Paterniti of UC Davis, and Deborah K. Orosz of Harvard University.
A grant
from the National Institute on Aging (5K12AG001004) funded the study.
The UCLA
Department of Family Medicine provides comprehensive primary care to entire
families, from newborns to seniors. It provides low-risk obstetrical services
and prenatal and inpatient care at UCLA Medical Center, Santa Monica, and
outpatient care at the University Family Health Center in Santa Monica and the
Mid-Valley Family Health Center, located in a Los Angeles County Health Center
in Van Nuys, Calif. The department is also a leader in family medicine
education, for both medical students and residents, and houses a significant
research unit focusing on health care disparities among immigrant families and
minority communities and other underserved populations in Los Angeles and
California.
Subscribe to:
Posts (Atom)